Designing a CPAP Cannula for Premature Babies
Dr. Mohamed, Vice Chair of Neonatology at the Cleveland Clinic Children’s Hospital and founder of CPAP Cannula, LLC, came to Root3 Labs with a big idea to develop a novel CPAP cannula for premature babies. He recognized the shortcomings with many CPAP cannula devices currently used in Neonatal Care Units (NICU). They can contain multiple components and end up being secured with rudimentary materials such as rubber bands and safety pins. Dr. Mohamed had a strong goal when partnering with us – to design a simple, comfortable, and user-friendly CPAP cannula for premature babies.
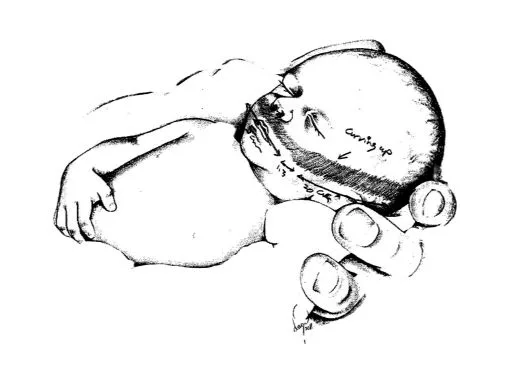
Initial sketch from Dr. Mohamed
Engineering Comfort for Premature Babies
Our minimum viable product (MVP) was easy to identify from the start: a single-piece cannula that would be easy to use and provide optimal comfort for premature babies. We were able to turn Dr. Mohamed’s initial sketches into a functional 3D model with CAD. Once the designs were modeled in CAD we could run Finite Element Analysis (FEA) and flow analysis testing. These test results were crucial to ensure our design was strong enough to support the weight of a baby’s head and provide enough airflow.
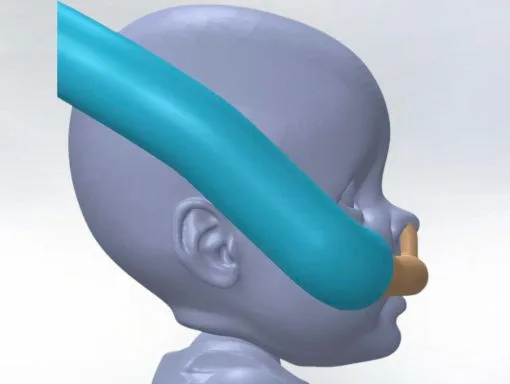
Initial design modeled in CAD
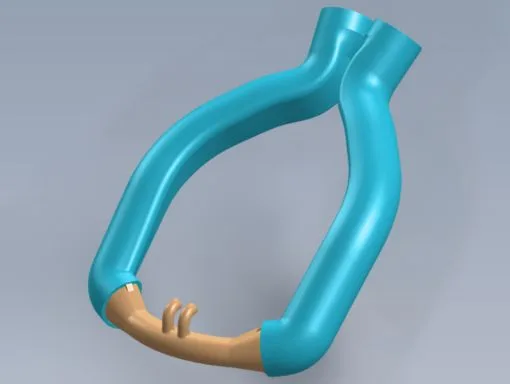
Initial design modeled in CAD
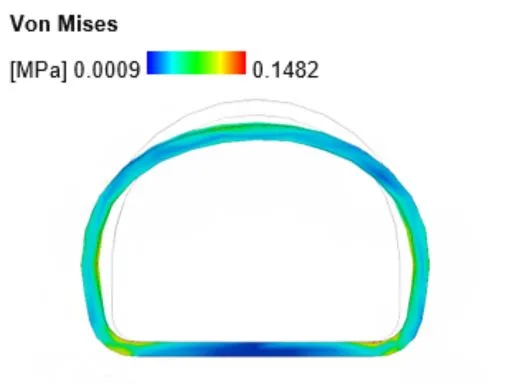
FEA test on initial prototype

Pressure testing
We used 3D printing to fabricate some initial test units for Dr. Mohamed to confirm fit and function. We then turned our attention to identifying a way to prototype and manufacture the device on a larger scale.
Regulatory Navigation and Clinical Trials
After exploring multiple fabrication methods, we found that dip molding was the best fit to scale up production. We designed mandrels in 7 different sizes and worked with a dip molding vendor to fabricate the devices. After progressing through a few design iterations to fine-tune the dimensions, several dozen devices were produced for clinical trials.
Meanwhile, Dr. Mohamed worked with a regulatory consultant to prepare the paperwork and testing needed to successfully gain FDA clearance. Root3 Labs assisted with some testing at this stage, as well. We were able to verify that the CPAP Cannula device would hold air pressure under the temperature and humidity conditions typically used in an infant incubator.

Mandrel used for dip molding
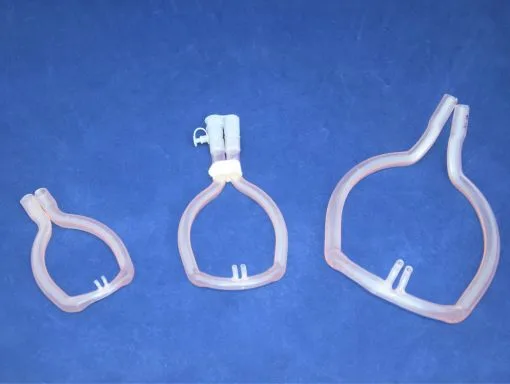
Final design in multiple sizes
Today Dr. Mohamed is diligently working towards bringing these innovative cannulas to hospitals. This collaborative effort promises to bring a breath of fresh air – literally and figuratively – to the lives of countless premature babies.
Stay tuned for updates on this impactful project, and visit Dr. Mohamed on LinkedIn to learn more about his work!
Highlights
DESIGN FOCUS
- Material Selection
- Manufacturability
- Ergonomics and Usability
FABRICATION
- Material Selection
- Dip Molding
- Safe for Premature Babies
